Decision-making about anticoagulation is among the most challenging aspects of atrial fibrillation (AF) management from the perspectives of both patients and physicians. On one hand, AF-related stroke, the most feared sequela of the arrhythmia, is more likely to be fatal or severely debilitating than strokes from other causes and can often be prevented with oral anticoagulants. On the other hand, anticoagulation causes major and minor bleeding, impacts quality of life, is costly to patients and the health care system, and has poor long-term compliance rates. Guidelines recommend lifelong anticoagulation on the basis of upstream risk factors irrespective of whether the AF burden is low from spontaneous termination or as the result of rhythm control strategies including antiarrhythmic drugs and ablation. This practice represents 1 example in medicine where identical treatment is administered without regard to the burden of disease or even in the face of disease diminution or resolution. Frequently cited reasons for this recommendation include the modest long-term success rates of rhythm control interventions, the high proportion of asymptomatic AF, and the uncertain role of the atrial myopathy that hypothetically may cause cardioembolic events independent of the arrhythmia. The rising prevalence of AF and the risks associated with this “1-size fits all” strategy make clear, however, that innovative approaches are needed and will have increasing importance over time.
Recent observations and developments may change this critical aspect of AF management. First, accumulating evidence supports the contention that AF is not a dichotomous variable and that the amount of AF, measured in duration or burden, plays a role in stroke risk. Indeed, data from pacemakers and defibrillators demonstrate that hours of AF are needed to increase stroke risk for most patients and that the risk waxes and wanes after an AF episode.1,2 Second, implantable devices and now consumer-grade digital health technologies capable of detecting AF allow for continuous, remote, long-term monitoring even in the absence of symptoms. Third, direct oral anticoagulants provide rapid-onset anticoagulation within a few hours of a single dose. Together, these advances allow for targeted, personalized, “pill-in-pocket” anticoagulation taken only for a limited time in response to a prolonged AF episode (Figure). Limiting anticoagulation exposure only to the high-risk period could protect against cardioembolic events and reduce bleeds, and achieve both goals at a reduced cost.
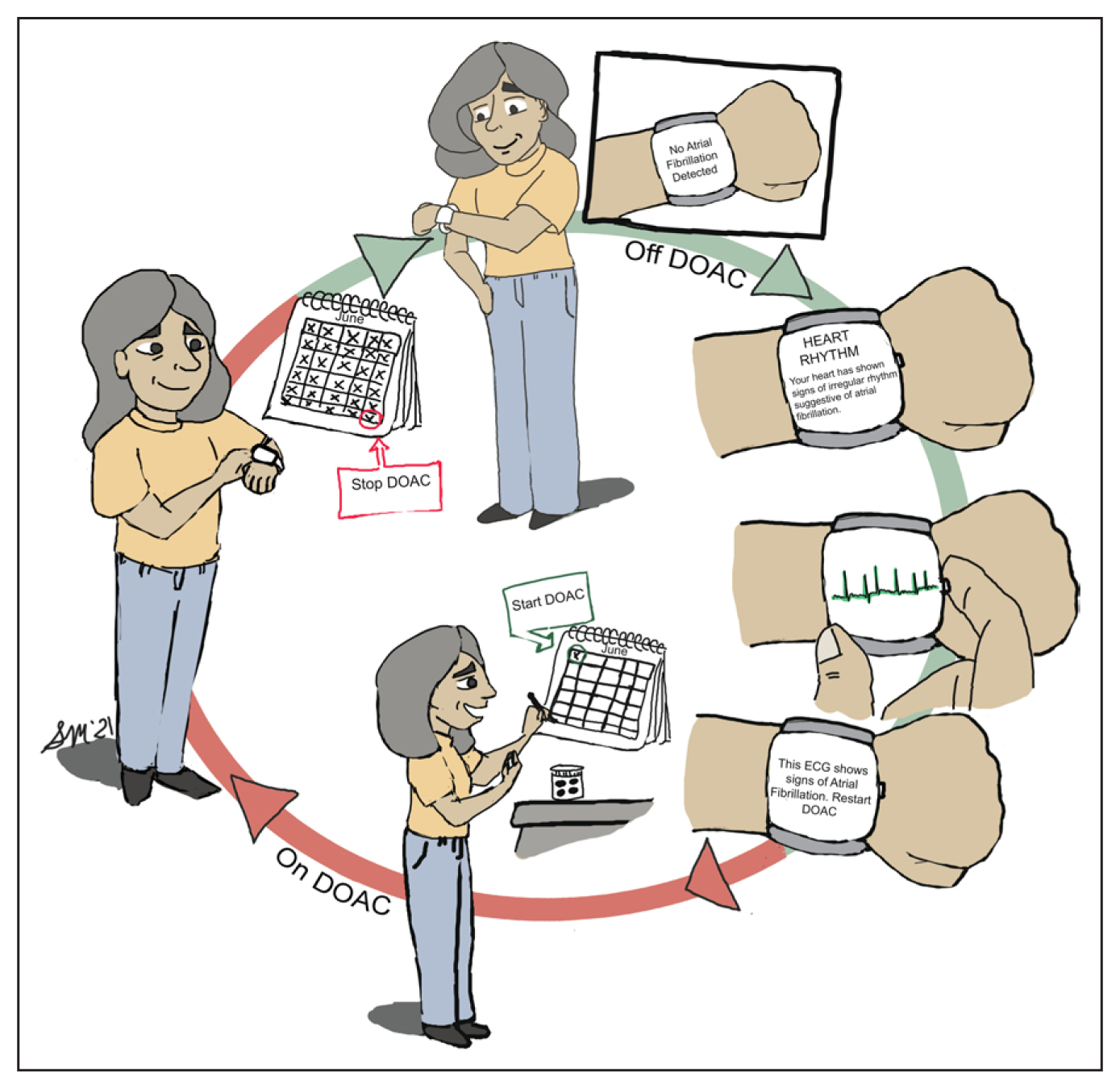
Figure. “Pill-in-pocket” anticoagulation.
Twelve o’clock: previously prescribed direct oral anticoagulant (DOAC) withheld while patient is in sinus rhythm as confirmed by the atrial fibrillation–sensing smartwatch; three o’clock: smartwatch alerts patient of irregular rhythm, atrial fibrillation confirmed by ECG, and patient instructed to resume DOAC; six o’clock: patient starts 30-day course of DOAC; nine o’clock: patient completes 30-day course of DOAC.
Two single-arm pilot studies have demonstrated the feasibility of this approach. REACT.COM (Rhythm Evaluation for Anticoagulation With Continuous Monitoring) and TACTIC-AF (Tailored Anticoagulation for Non-Continuous Atrial Fibrillation) used continuous remote monitoring from insertable cardiac monitors and dual chamber pacemakers or defibrillators, respectively, to reinitiate anticoagulation for 30 days after an AF episode of prespecified duration. In REACT.COM, a 94% reduction in anticoagulation use was observed using a 1-hour-duration threshold for anticoagulation reinitiation.3 TACTIC-AF observed a 75% reduction in time on anticoagulation using a threshold of 6 minutes or total burden >6 h/d.4 Combined, these studies enrolled 96 patients with 112 patient-years of follow-up and observed no strokes, suggesting that the concept of “pill-in-pocket” anticoagulation is no longer fiction. Most patients, however, have no indication for pacemakers or defibrillators, and insertable cardiac monitors are invasive and expensive. All of these devices are also physician-facing and require a significant infrastructure to receive and adjudicate the data and respond to the patient in a timely manner. If this form of precision medicine is to be adopted by the tens of millions of individuals with AF worldwide, we must first prove that this approach is both safe and effective using a noninvasive, inexpensive, patient-facing AF monitoring system available to all.
With more than three-quarters of the population owning a smartphone, digital health technologies are positioned to play a central role in AF detection and management. Wearable devices capable of pulse detection became popular in the late 1970s for use in athletic training, and wrist-worn devices that use photoplethysmography can passively monitor heart rate and rhythm for long periods at low cost. Multiple studies evaluating the AF algorithms on these devices have consistently demonstrated sensitivities and specificities >95%. Devices from multiple manufacturers now also provide for the recording of a confirmatory singlelead ECG with automated rhythm adjudication. The use of these smartwatches with passive irregular rhythm monitor and on-demand ECG confirmation now allows the “pill-in-pocket” concept to be evaluated in the population at large.
Why might this approach be problematic? To be effective, the strategy hinges on several assertions, including the recognition that AF itself causes cardioembolic stroke, that stroke risk is low during long periods of sinus rhythm, and that stroke is temporally related to an AF episode. Although data from clinical trials and observational studies support these contentions, controversy exists. Some studies examining the temporal relationship between AF and stroke show that neurological events occurred well before or after an AF episode and sometimes in the absence of AF altogether.5 It is worth noting, however, that many of these AF episodes were brief, and no effort was made to prospectively adjudicate stroke mechanism. This last point deserves emphasis because not all strokes are a result of AF, even in those with an AF history, a fact especially relevant in patients with multiple stroke risk factors like those enrolled in these studies.
Perhaps even more foolish than challenging the status quo is continuing to treat all patients the same while ignoring advances that provide an opportunity to change a practice unacceptable to so many. Although guidelines are clear as to who should receive anticoagulation, the truth is that doctors often fail to prescribe these drugs, patients often choose not to take them, and both groups routinely question our generic approach to anticoagulation therapy in the age of personalized medicine. There is now a strong rationale for leveraging recent insights into the relationship between AF and stroke and breakthroughs in technology and pharmacology to test a potential paradigm shift in treatment. Given the growing enormity of the AF population and the magnitude of the issues at stake, it is time for a pivotal randomized trial to determine whether “pill-in-pocket” anticoagulation can finally become fact.
REFERENCES
Kaplan RM, Koehler J, Ziegler PD, Sarkar S, Zweibel S, Passman RS. Stroke risk as a function of atrial fibrillation duration and CHA2DS2-VASc score. Circulation. 2019;140:1639–1646. doi: 10.1161/CIRCULATIONAHA.119.041303 | |
Turakhia MP, Ziegler PD, Schmitt SK, Chang Y, Fan J, Than CT, Keung EK, Singer DE. Atrial fibrillation burden and short-term risk of stroke: case-crossover analysis of continuously recorded heart rhythm from cardiac electronic implanted devices. Circ Arrhythm Electrophysiol. 2015;8:1040–1047 doi: 10.1161/CIRCEP.114.003057 | |
Passman R, Leong-Sit P, Andrei AC, Huskin A, Tomson TT, Bernstein R, Ellis E, Waks JW, Zimetbaum P. Targeted anticoagulation for atrial fibrillation guided by continuous rhythm assessment with an insertable cardiac monitor: the Rhythm Evaluation for Anticoagulation With Continuous Monitoring (REACT.COM) pilot study. J Cardiovasc Electrophysiol. 2016;27:264–270. doi: 10.1111/jce.12864 | |
Waks JW, Passman RS, Matos J, Reynolds M, Thosani A, Mela T, Pederson D, Glotzer TV, Zimetbaum P. Intermittent anticoagulation guided by continuous atrial fibrillation burden monitoring using dualchamber pacemakers and implantable cardioverter-defibrillators: Results from the Tailored Anticoagulation for Non-Continuous Atrial Fibrillation (TACTIC-AF) pilot study. Heart Rhythm. 2018;15:1601–1607. doi: 10.1016/j.hrthm.2018.06.027 | |
Brambatti M, Connolly SJ, Gold MR, Morillo CA, Capucci A, Muto C, Lau CP, Van Gelder IC, Hohnloser SH, Carlson M, et al; ASSERT Investigators. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation. 2014;129:2094–2099. doi: 10.1161/CIRCULATIONAHA.113.007825 |